What is FDA Approved Plastic? Key Insights for OEM Managers
Overview
FDA-approved plastic is all about safety and trust. These materials have gone through thorough testing by the U.S. Food and Drug Administration, making sure they’re safe for products that touch our food, medicines, or medical devices. This approval is crucial because it not only helps reduce contamination risks but also boosts the credibility of OEM products. When a product complies with FDA regulations, it builds consumer trust and lowers liability risks.
Now, you might be wondering how this affects you. Well, think about it: when you see that FDA approval, doesn’t it make you feel more confident in what you’re buying? It’s like a stamp of assurance that says, “Hey, this is safe!” Plus, for manufacturers, it’s a game-changer in the market. With the right approvals, they can stand out and show customers they care about quality and safety.
So, next time you’re considering a product, keep an eye out for that FDA approval. It’s not just a label; it’s a commitment to safety and quality that benefits everyone involved.
Introduction
Navigating the world of FDA-approved plastics can feel like a maze for manufacturers, especially when it comes to food and medical device production. These materials, cleared by the U.S. Food and Drug Administration, not only meet tough safety standards but also boost marketability and consumer confidence.
But here’s the kicker: as compliance gets trickier, how can OEM managers make sure they're using these materials effectively? It’s all about protecting public health while keeping that competitive edge, right? Let's dive into this together!
Defining FDA Approved Plastics: Key Concepts and Significance
FDA approved plastic is a type of material that has passed a thorough check by the U.S. Food and Drug Administration (FDA) for use in products that touch our food, medicines, or medical devices. Why does this matter? Because FDA approval is a big deal! It means these materials meet strict safety and health standards, which helps reduce risks like contamination or bad reactions. For manufacturers in the OEM sector, this approval is crucial since it directly impacts safety, compliance, and how well their products sell.
Let’s look at some real-world examples. Think about polyethylene and polypropylene. These plastics are often found in medical devices like syringes and IV bags that are vital for patient care. When the FDA gives a thumbs up to FDA approved plastic, it not only ensures their safety but also builds trust in the products that use them.
But there’s more to FDA approval than just ticking boxes for compliance. It can actually give OEM manufacturers a leg up in the market. Knowing that their products meet regulatory standards can really influence the buying decisions of healthcare providers and consumers. Industry leaders emphasize that FDA approval isn’t just a hurdle to jump over; it’s a key part of development that fosters trust and reliability in the medical and food industries.
To wrap it up, the use of for synthetic materials is a must in both food and medical fields. It ensures that substances are safe for consumers and meet regulatory standards. This not only protects public health but also boosts the reputation and marketability of OEM products. So, next time you think about FDA approval, remember—it’s not just about regulations; it’s about safety and trust!
Understanding FDA Regulations: Compliance and Standards for Plastics
When it comes to FDA regulations on polymers, particularly FDA approved plastic, it can feel a bit overwhelming, right? These rules are mainly laid out in Title 21 of the Code of Federal Regulations (CFR), which specifies that materials such as FDA approved plastic are acceptable for things like food contact substances and medical devices. To stick to these regulations, thorough testing and detailed documentation are a must. After all, nobody wants plastics leaking harmful substances into their products.
You might be surprised to learn that many manufacturers find compliance challenging. In fact, the second most common FDA citation is all about reviewing production records, making up 31% of observations from 2016 to 2020. This really highlights how crucial it is for manufacturers to have tight review procedures and solid documentation in place. It’s not just about avoiding recalls and penalties; it’s about protecting your reputation, too.
Now, let’s talk about staying updated. The regulatory landscape is always changing, and it’s essential for manufacturers to keep up. Compliance with Title 21 CFR isn’t just a box to check; it’s vital for ensuring safety and market success. At Lincoln Plastics, we work hand-in-hand with OEMs to make sure their products meet all the necessary standards. This includes specific checks for ‘fit and function’ and sourcing colors that match your Pantone specifications for consistency.
Our assurance system is robust, featuring a dedicated reference manual for your polymer profile. This includes drawings, essential in-process checks, and run documentation, ensuring your rigid profiles hit all the critical dimensions. Understanding these compliance standards—like adopting a and creating documented policies for operational systems—is key for OEM managers. It not only helps protect your products but also boosts efficiency. So, how are you planning to tackle these compliance challenges?
Types of FDA Approved Plastics: Applications and Characteristics
Did you know there are various FDA-approved plastic materials available, each with its own unique features tailored for specific uses? For instance, you might be familiar with polyethylene (PE), polypropylene (PP), and polyvinyl chloride (PVC). Polyethylene is a popular choice for food packaging because of its fantastic moisture barrier properties. On the flip side, polypropylene stands out for its high melting point and chemical resistance, making it perfect for medical applications. And let’s not forget PVC, which is often used in medical tubing and containers.
At Lincoln Plastics, we work closely with OEMs to ensure these materials meet all necessary standards. We even conduct special checks for ‘fit and function’ using various types of check gauges to guarantee a proper end-use fit. You might be wondering about —no worries! We can easily source colors that match your Pantone specifications across different manufacturers.
Our robust assurance system includes essential in-process evaluations and documentation. This helps OEM managers make informed decisions about which materials, specifically FDA-approved plastic, to use in their products, ensuring everything is accurate and compliant. So, whether you're selecting materials for packaging or medical uses, we’re here to help you every step of the way!
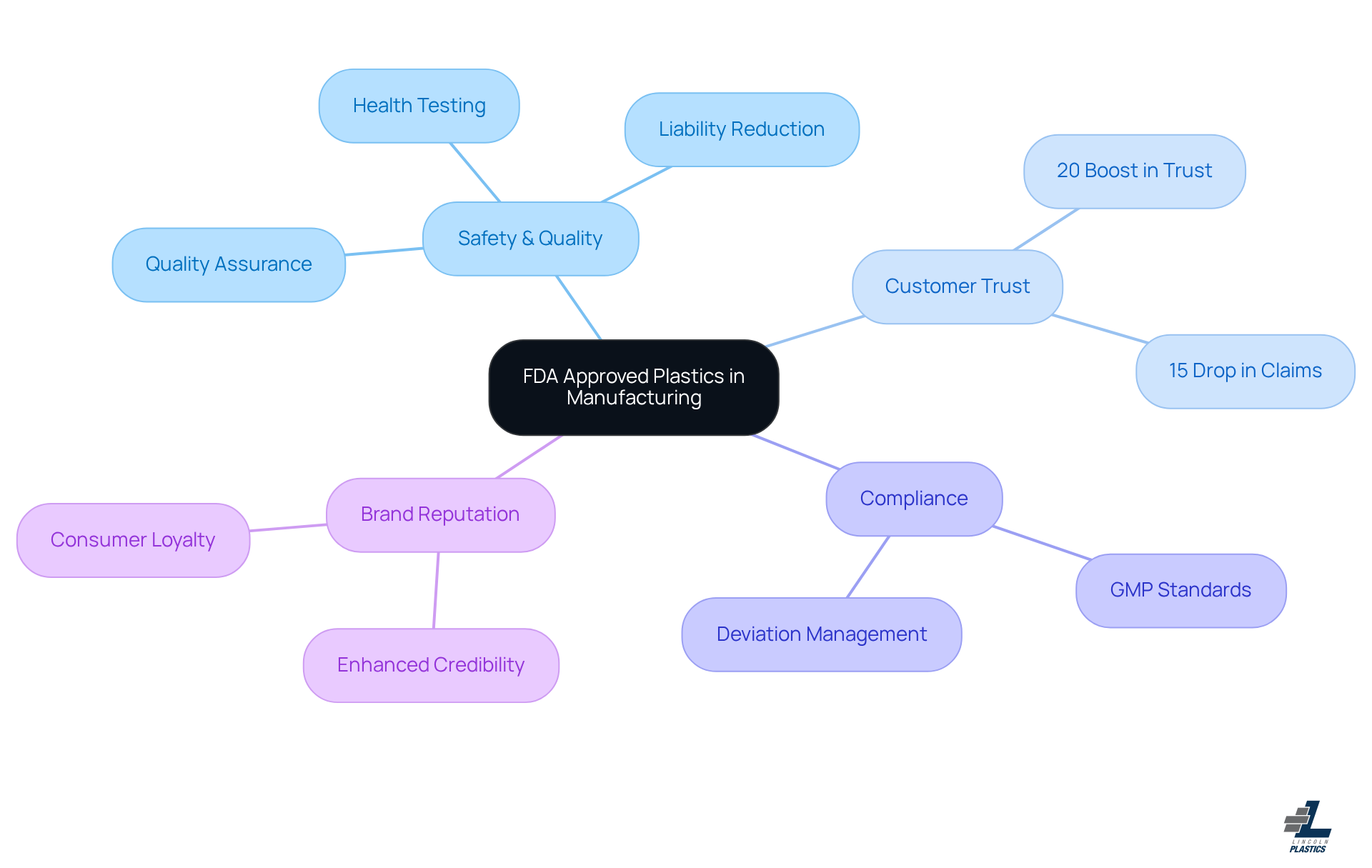
The Importance of FDA Approved Plastics in Manufacturing: Safety and Quality Assurance
Using FDA-approved plastic materials in manufacturing is super important for ensuring safety and quality. These materials go through tough testing to make sure they don’t pose health risks, which is especially crucial in industries like food and pharmaceuticals. By choosing FDA-approved plastic, manufacturers can enhance their credibility and reduce liability risks associated with safety. This commitment to compliance builds trust and loyalty among consumers, which can really enhance a brand's reputation and competitive edge in the market.
Now, you might be wondering how this affects customer satisfaction. Well, industry experts highlight that using can lead to happier customers and increased sales. People tend to choose products that prioritize their health and safety. For example, a recent study found that companies using FDA-approved plastic materials saw a 20% boost in customer trust and a 15% drop in product liability claims.
At Lincoln Plastics, we work closely with OEMs to ensure our materials are made from FDA-approved plastic that meets all standards, including specific tests for ‘fit and function.’ Our quality system is robust, featuring a dedicated quality book for each plastic profile, complete with drawings, critical in-process checks, and run documentation.
Experts from the FDA Group stress the importance of staying compliant to dodge potential legal issues. They say, 'Establishing clear policies and SOPs for deviation management is crucial for providing a consistent framework for handling deviations.' This really highlights how vital FDA compliance is for protecting both consumer health and manufacturer interests.
Conclusion
FDA approved plastics are super important for keeping our food and medical products safe and of high quality. They go through tough testing and meet strict standards, which not only protects our health but also boosts the reputation of manufacturers. When you see that approval, it means these materials have passed rigorous safety checks, building trust with consumers and healthcare providers.
Now, let’s dive into why understanding FDA regulations is crucial. There are different types of FDA approved plastics, each with its own uses. Manufacturers need to navigate this complex landscape of compliance to steer clear of legal troubles and keep their products top-notch. By using FDA approved plastics, OEM managers can really amp up their market appeal and customer satisfaction, leading to better sales and less risk of liability.
In a world where health and safety are key, choosing FDA approved materials isn’t just about following rules; it’s a smart move. Manufacturers should stay updated on compliance standards and invest in quality assurance processes. This commitment not only safeguards public health but also builds brand loyalty and strengthens competitive positioning. So, if you're an OEM looking to succeed in today’s challenging market, embracing FDA approved plastics is essential!
Frequently Asked Questions
What is FDA approved plastic?
FDA approved plastic is a material that has been thoroughly evaluated by the U.S. Food and Drug Administration (FDA) for safety in products that come into contact with food, medicines, or medical devices.
Why is FDA approval important?
FDA approval is significant because it ensures that materials meet strict safety and health standards, reducing risks of contamination or adverse reactions. It is crucial for manufacturers as it impacts safety, compliance, and product sales.
Can you provide examples of FDA approved plastics?
Examples of FDA approved plastics include polyethylene and polypropylene, which are commonly used in medical devices such as syringes and IV bags.
How does FDA approval affect OEM manufacturers?
FDA approval can give OEM manufacturers a competitive advantage in the market, as it influences the purchasing decisions of healthcare providers and consumers by fostering trust and reliability in their products.
What is the significance of FDA approval in the food and medical industries?
The use of FDA approved plastic is essential in the food and medical sectors because it ensures that materials are safe for consumers, meet regulatory standards, and enhance the reputation and marketability of OEM products.
List of Sources
- Defining FDA Approved Plastics: Key Concepts and Significance
- Overview of Device Regulation (https://fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation)
- FDA Testing and Approval Processes for Implantable Devices Made from PEEK Polymer - Part 2 (https://genesismedicalplastics.com/fda-testing-approval-processes-implantable-devices-made-from-peek-polymer-part-2)
- Premarket Notification 510(k) (https://fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k)
- Understanding FDA Regulations: Compliance and Standards for Plastics
- 5 Common 21 CFR Non-Compliance Violations, and How to Avoid Them (https://mddionline.com/manufacturing/top-5-crf-21-non-compliance-citations-and-how-to-avoid-them)
- CFR - Code of Federal Regulations Title 21 – FDA (https://nsf.org/knowledge-library/fda-recent-amendment-cfr-title-21)
- Understanding FDA and CFR Regulations: What They are and Why They’re Important in Life Sciences | Accruent (https://accruent.com/resources/blog-posts/understanding-fda-cfr-regulations-what-they-are-why-theyre-important-life)
- Top 2 FDA Citations to Avoid - PSC Biotech® (https://biotech.com/2022/01/03/top-fda-citations-to-avoid)
- The Importance of FDA Approved Plastics in Manufacturing: Safety and Quality Assurance
- Deviation Management in the FDA-Regulated Industries: Basics and Best Practices (https://thefdagroup.com/blog/deviation-management)
- What is GMP | Good Manufacturing Practices | SafetyCulture (https://safetyculture.com/topics/gmp)
- US FDA CDRH 2024 Annual Report Summary (https://emergobyul.com/news/us-fda-cdrh-2024-annual-report-summary)


